Microsclerotherapy Treatment Is Revolutionizing the Therapies Related To Vein Disorder Such As Varicose Veins in Healthcare Sectors across the Globe
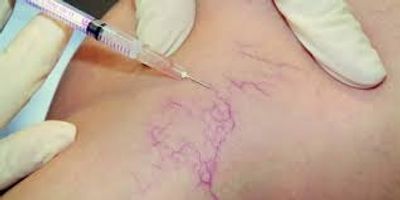
Microsclerotherapy treatment is being practiced by medical professionals all over the world especially in regions such as U.S., U.K., and Italy. Microsclerotherapy treatment is a medical procedure in which a fine needle is introduced into the vein to deliver a low-pressure spray containing medications designed to break up the blockage of the internal lining of the vein. The microsclerotia agent is injected into the vein so that it can break up the clots that form and cause pain and discomfort. These clots are called varicose veins and are caused by scar tissue build-up due to past events or simply aging. Microsclerotherapy treatment has been effective in treating some varicose veins, as well as spider veins.
There
are many benefits to microsclerotherapy treatment besides removing spider and
varicose veins. This procedure can be used for superficial disruptions of blood
vessels such as varicose veins. However, to treat spider veins, the doctor must
first inject a corticosteroid into the vessel beforehand. The main advantage of
microsclerotherapy treatment is that the sclerosing agent works deep within the
layers of the skin and not just on the surface. The treatment can be very
effective for those suffering from varicose veins as well as for spider veins,
even for those with severe symptoms.
U.S.
Department of Health and Human Services stated that over 30 million people in
the U.S. are diagnosed with venous diseases such as varicose veins and chronic
venous insufficiency (CVI), which is a more serious form of venous disease. Varicose
veins and spider veins are not a rare disease and 50% to 55% of women and 40%
to 45% of men suffer from vein diseases. However, among the 30 million
diagnosed cases approximately only 1.9 million opt for treatments such as Microsclerotherapy
treatment. So pharmaceutical firms are launching new treatment systems in the
medical sector. For instance, in February 2019, Medtronic launched the Accurian
RF ablation system in the United States after getting approval from the U.S.
Food and Drug Association (FDA).
The
ablation platform of Accurian radiofrequency (RF) combines quad-core processing
and hardware designs, which result in a complex algorithm that controls the
temperature and manages the power during ablation surgery. Cardiac ablation
surgery is a process to destroy or scar the cardiac tissues, which are enabling
incorrect electrical signals to produce abnormal heart rate. The Accurian
radiofrequency (RF) ablation platform can consists of three types of ablation
procedures, i.e., enhanced, standard and pulsed.

Comments
Post a Comment